Syphilis
| Syphilis | |
|---|---|
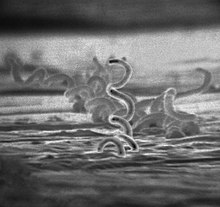 | |
| Electron micrograph of Treponema pallidum bacteria | |
| Specialty | Infectious disease |
| Symptoms | Firm, painless, non-itchy skin ulcer[1] |
| Causes | Treponema pallidum, usually spread by sex[1] |
| Diagnostic method | Blood tests, dark field microscopy of infected fluid[2][3] |
| Differential diagnosis | Many other diseases[2] |
| Prevention | Condoms, Long-term monogamous relationships[2] |
| Treatment | Antibiotics[4] |
| Frequency | 45.4 million / 0.6% (2015, global)[5] |
| Deaths | 107,000 (2015, global)[6] |
Syphilis (/ˈsɪfəlɪs/) is a sexually transmitted infection caused by the bacterium Treponema pallidum subspecies pallidum.[1] The signs and symptoms depend on the stage it presents: primary, secondary, latent or tertiary.[1][2] The primary stage classically presents with a single chancre (a firm, painless, non-itchy skin ulceration usually between 1 cm and 2 cm in diameter) though there may be multiple sores.[2] In secondary syphilis, a diffuse rash occurs, which frequently involves the palms of the hands and soles of the feet.[2] There may also be sores in the mouth or vagina.[2] Latent syphilis has no symptoms and can last years.[2] In tertiary syphilis, there are gummas (soft, non-cancerous growths), neurological problems, or heart symptoms.[3] Syphilis has been known as "the great imitator" because it may cause symptoms similar to many other diseases.[2][3]

Syphilis is most commonly spread through sexual activity.[2] It may also be transmitted from mother to baby during pregnancy or at birth, resulting in congenital syphilis.[2][7] Other diseases caused by Treponema bacteria include yaws (T. pallidum subspecies pertenue), pinta (T. carateum), and nonvenereal endemic syphilis (T. pallidum subspecies endemicum).[3] These three diseases are not typically sexually transmitted.[8] Diagnosis is usually made by using blood tests; the bacteria can also be detected using dark field microscopy.[2] The Centers for Disease Control and Prevention (U.S.) recommends for all pregnant women to be tested.[2]
The risk of sexual transmission of syphilis can be reduced by using a latex or polyurethane condom.[2] Syphilis can be effectively treated with antibiotics.[4] The preferred antibiotic for most cases is benzathine benzylpenicillin injected into a muscle.[4] In those who have a severe penicillin allergy, doxycycline or tetracycline may be used.[4] In those with neurosyphilis, intravenous benzylpenicillin or ceftriaxone is recommended.[4] During treatment people may develop fever, headache, and muscle pains, a reaction known as Jarisch–Herxheimer.[4]
In 2015, about 45.4 million people had syphilis infections,[5] of which six million were new cases.[9] During 2015, it caused about 107,000 deaths, down from 202,000 in 1990.[6][10] After decreasing dramatically with the availability of penicillin in the 1940s, rates of infection have increased since the turn of the millennium in many countries, often in combination with human immunodeficiency virus (HIV).[3][11] This is believed to be partly due to unsafe drug use, increased prostitution, and decreased use of condoms.[12][13][14]
Signs and symptoms
Syphilis can present in one of four different stages: primary, secondary, latent, and tertiary, and may also occur congenitally.[15] There may be no symptoms.[16] It was referred to as "the great imitator" by Sir William Osler due to its varied presentations.[3][17][18]
Primary

Primary syphilis is typically acquired by direct sexual contact with the infectious lesions of another person.[19] Approximately 2–6 weeks after contact (with a range of 10–90 days) a skin lesion, called a chancre, appears at the site and this contains infectious bacteria.[20][21] This is classically (40% of the time) a single, firm, painless, non-itchy skin ulceration with a clean base and sharp borders approximately 0.3–3.0 cm in size.[3] The lesion may take on almost any form.[22] In the classic form, it evolves from a macule to a papule and finally to an erosion or ulcer.[22] Occasionally, multiple lesions may be present (~40%),[3] with multiple lesions being more common when coinfected with HIV.[22] Lesions may be painful or tender (30%), and they may occur in places other than the genitals (2–7%).[22] The most common location in women is the cervix (44%), the penis in heterosexual men (99%), and anally and rectally in men who have sex with men (34%).[22] Lymph node enlargement frequently (80%) occurs around the area of infection,[3] occurring seven to 10 days after chancre formation.[22] The lesion may persist for three to six weeks if left untreated.[3]
Secondary


Secondary syphilis occurs approximately four to ten weeks after the primary infection.[3] While secondary disease is known for the many different ways it can manifest, symptoms most commonly involve the skin, mucous membranes, and lymph nodes.[23] There may be a symmetrical, reddish-pink, non-itchy rash on the trunk and extremities, including the palms and soles.[3][24] The rash may become maculopapular or pustular.[3] It may form flat, broad, whitish, wart-like lesions on mucous membranes, known as condyloma latum.[3] All of these lesions harbor bacteria and are infectious.[3] Other symptoms may include fever, sore throat, malaise, weight loss, hair loss, and headache.[3] Rare manifestations include liver inflammation, kidney disease, joint inflammation, periostitis, inflammation of the optic nerve, uveitis, and interstitial keratitis.[3][25] The acute symptoms usually resolve after three to six weeks;[25] about 25% of people may present with a recurrence of secondary symptoms.[23][26] Many people who present with secondary syphilis (40–85% of women, 20–65% of men) do not report previously having had the classical chancre of primary syphilis.[23]
Latent
Latent syphilis is defined as having serologic proof of infection without symptoms of disease.[19] It develops after secondary syphilis and is divided into early latent and late latent stages.[27] Early latent syphilis is defined by the World Health Organization as less than 2 years after original infection.[27] Early latent syphilis is infectious as up to 25% of people can develop a recurrent secondary infection (during which bacteria are actively replicating and are infectious).[27] Two years after the original infection the person will enter late latent syphilis and is not as infectious as the early phase.[25][28] The latent phase of syphilis can last many years after which, without treatment, approximately 15-40% of people can develop tertiary syphilis.[29]
Tertiary

Tertiary syphilis may occur approximately 3 to 15 years after the initial infection and may be divided into three different forms: gummatous syphilis (15%), late neurosyphilis (6.5%), and cardiovascular syphilis (10%).[3][25] Without treatment, a third of infected people develop tertiary disease.[25] People with tertiary syphilis are not infectious.[3]
Gummatous syphilis or late benign syphilis usually occurs 1 to 46 years after the initial infection, with an average of 15 years.[3] This stage is characterized by the formation of chronic gummas, which are soft, tumor-like balls of inflammation which may vary considerably in size.[3] They typically affect the skin, bone, and liver, but can occur anywhere.[3]
Cardiovascular syphilis usually occurs 10–30 years after the initial infection.[3] The most common complication is syphilitic aortitis, which may result in aortic aneurysm formation.[3]
Neurosyphilis refers to an infection involving the central nervous system. Involvement of the central nervous system in syphilis (either asymptomatic or symptomatic) can occur at any stage of the infection.[21] It may occur early, being either asymptomatic or in the form of syphilitic meningitis; or late as meningovascular syphilis, manifesting as general paresis or tabes dorsalis.[3]
Meningovascular syphilis involves inflammation of the small and medium arteries of the central nervous system. It can present between 1–10 years after the initial infection. Meningovascular syphilis is characterized by stroke, cranial nerve palsies and spinal cord inflammation.[30] Late symptomatic neurosyphilis can develop decades after the original infection and includes 2 types; general paresis and tabes dorsalis. General paresis presents with dementia, personality changes, delusions, seizures, psychosis and depression.[30] Tabes dorsalis is characterized by gait instability, sharp pains in the trunk and limbs, impaired positional sensation of the limbs as well as having a positive Romberg's sign.[30] Both tabes dorsalis and general paresis may present with Argyll Robertson pupil which are pupils that constrict when the person focuses on near objects (accommodation reflex) but do not constrict when exposed to bright light (pupillary reflex).
Congenital
Congenital syphilis is that which is transmitted during pregnancy or during birth.[7] Two-thirds of syphilitic infants are born without symptoms.[7] Common symptoms that develop over the first couple of years of life include enlargement of the liver and spleen (70%), rash (70%), fever (40%), neurosyphilis (20%), and lung inflammation (20%).[7] If untreated, late congenital syphilis may occur in 40%, including saddle nose deformation, Higouménakis' sign, saber shin, or Clutton's joints among others.[7] Infection during pregnancy is also associated with miscarriage.[31] The main dental defects seen in congenital syphilis are the peg-shaped, notched incisors known as Hutchinson's teeth and so-called mulberry molars (also known as Moon or Fournier molars), defective permanent molars with rounded, deformed crowns resembling a mulberry.[32]
Cause
Bacteriology

Treponema pallidum subspecies pallidum is a spiral-shaped, Gram-negative, highly mobile bacterium.[11][22] Two other human diseases are caused by related Treponema pallidum subspecies, yaws (subspecies pertenue) and bejel (subspecies endemicum), and one further caused by the very closely related Treponema carateum, pinta.[3][33] Unlike subspecies pallidum, they do not cause neurological disease.[7] Humans are the only known natural reservoir for subspecies pallidum.[34] It is unable to survive more than a few days without a host.[22] This is due to its small genome (1.14Mbp) failing to encode the metabolic pathways necessary to make most of its macronutrients.[22] It has a slow doubling time of greater than 30 hours.[22] The bacterium is known for its ability to evade the immune system and its invasiveness.[35]
Transmission
Syphilis is transmitted primarily by sexual contact or during pregnancy from a mother to her baby; the bacterium is able to pass through intact mucous membranes or compromised skin.[3][34] It is thus transmissible by kissing near a lesion, as well as manual, oral, vaginal, and anal sex.[3][36][37] Approximately 30% to 60% of those exposed to primary or secondary syphilis will get the disease.[25] Its infectivity is exemplified by the fact that an individual inoculated with only 57 organisms has a 50% chance of being infected.[22] Most new cases in the United States (60%) occur in men who have sex with men; and in this population 20% of syphilis cases were due to oral sex alone.[3][36] Syphilis can be transmitted by blood products, but the risk is low due to screening of donated blood in many countries.[3] The risk of transmission from sharing needles appears to be limited.[3]
It is not generally possible to contract syphilis through toilet seats, daily activities, hot tubs, or sharing eating utensils or clothing.[38] This is mainly because the bacteria die very quickly outside of the body, making transmission by objects extremely difficult.[39]
Diagnosis


Syphilis is difficult to diagnose clinically during early infection.[22] Confirmation is either via blood tests or direct visual inspection using dark field microscopy.[3][41] Blood tests are more commonly used, as they are easier to perform.[3] Diagnostic tests are unable to distinguish between the stages of the disease.[42]
Blood tests
Blood tests are divided into nontreponemal and treponemal tests.[22]
Nontreponemal tests are used initially and include venereal disease research laboratory (VDRL) and rapid plasma reagin (RPR) tests. False positives on the nontreponemal tests can occur with some viral infections, such as varicella (chickenpox) and measles. False positives can also occur with lymphoma, tuberculosis, malaria, endocarditis, connective tissue disease, and pregnancy.[19]
Because of the possibility of false positives with nontreponemal tests, confirmation is required with a treponemal test, such as Treponema pallidum particle agglutination assay (TPHA) or fluorescent treponemal antibody absorption test (FTA-Abs).[3] Treponemal antibody tests usually become positive two to five weeks after the initial infection[22] and remain positive for many years.[43] Neurosyphilis is diagnosed by finding high numbers of leukocytes (predominately lymphocytes) and high protein levels in the cerebrospinal fluid in the setting of a known syphilis infection.[3][19]
Direct testing
Dark field microscopy of serous fluid from a chancre may be used to make an immediate diagnosis.[22] Hospitals do not always have equipment or experienced staff members, and testing must be done within 10 minutes of acquiring the sample.[22] Two other tests can be carried out on a sample from the chancre: direct fluorescent antibody (DFA) and polymerase chain reaction (PCR) tests.[22] DFA uses antibodies tagged with fluorescein, which attach to specific syphilis proteins, while PCR uses techniques to detect the presence of specific syphilis genes.[22] These tests are not as time-sensitive, as they do not require living bacteria to make the diagnosis.[22]
Prevention
Vaccine
As of 2018[update], there is no vaccine effective for prevention.[34] Several vaccines based on treponemal proteins reduce lesion development in an animal model but research continues.[44][45]
Sex
Condom use reduces the likelihood of transmission during sex, but does not eliminate the risk.[46] The Centers for Disease Control and Prevention (CDC) states, "Correct and consistent use of latex condoms can reduce the risk of syphilis only when the infected area or site of potential exposure is protected.[47] However, a syphilis sore outside of the area covered by a latex condom can still allow transmission, so caution should be exercised even when using a condom."[48]
Abstinence from intimate physical contact with an infected person is effective at reducing the transmission of syphilis. The CDC states, "The surest way to avoid transmission of sexually transmitted diseases, including syphilis, is to abstain from sexual contact or to be in a long-term mutually monogamous relationship with a partner who has been tested and is known to be uninfected."[48]
Congenital disease

Congenital syphilis in the newborn can be prevented by screening mothers during early pregnancy and treating those who are infected.[50] The United States Preventive Services Task Force (USPSTF) strongly recommends universal screening of all pregnant women,[51] while the World Health Organization (WHO) recommends all women be tested at their first antenatal visit and again in the third trimester.[52][53] If they are positive, it is recommended their partners also be treated.[52] Congenital syphilis is still common in the developing world, as many women do not receive antenatal care at all, and the antenatal care others receive does not include screening.[50][54] It still occasionally occurs in the developed world, as those most likely to acquire syphilis are least likely to receive care during pregnancy.[50] Several measures to increase access to testing appear effective at reducing rates of congenital syphilis in low- to middle-income countries.[52] Point-of-care testing to detect syphilis appeared to be reliable, although more research is needed to assess its effectiveness and into improving outcomes in mothers and babies.[55]
Screening
The CDC recommends that sexually active men who have sex with men be tested at least yearly.[56] The USPSTF also recommends screening among those at high risk.[57]
Syphilis is a notifiable disease in many countries, including Canada,[58] the European Union,[59] and the United States.[60] This means health care providers are required to notify public health authorities, which will then ideally provide partner notification to the person's partners.[61] Physicians may also encourage patients to send their partners to seek care.[62] Several strategies have been found to improve follow-up for STI testing, including email and text messaging of reminders for appointments.[63]
Treatment
Historic use of mercury
As a form of chemotherapy, elemental mercury had been used to treat skin diseases in Europe as early as 1363.[64] As syphilis spread, preparations of mercury were among the first medicines used to combat it. Mercury is in fact highly anti-microbial: by the 16th century it was sometimes found to be sufficient to halt development of the disease when applied to ulcers as an inunction or when inhaled as a suffumigation. It was also treated by ingestion of mercury compounds.[65] Once the disease had gained a strong foothold, however, the amounts and forms of mercury necessary to control its development exceeded the human body's ability to tolerate it, and the treatment became worse and more lethal than the disease. Nevertheless, medically directed mercury poisoning became widespread through the 17th, 18th, and 19th centuries in Europe, North America, and India.[66] Mercury salts such as mercury (II) chloride were still in prominent medical use as late as 1916, and considered effective and worthwhile treatments.[67]
Early infections
The first-line treatment for uncomplicated syphilis (primary or secondary stages) remains a single dose of intramuscular benzathine benzylpenicillin.[68] The bacterium is highly vulnerable to penicillin when treated early, and a treated individual is typically rendered non-infective in about 24 hours.[69] Doxycycline and tetracycline are alternative choices for those allergic to penicillin; due to the risk of birth defects, these are not recommended for pregnant women.[68] Resistance to macrolides, rifampicin, and clindamycin is often present.[34] Ceftriaxone, a third-generation cephalosporin antibiotic, may be as effective as penicillin-based treatment.[3] It is recommended that a treated person avoid sex until the sores are healed.[38] In comparison to azithromycin for treatment in early infection, there is lack of strong evidence for superiority of azithromycin to benzathine penicillin G.[70]
Late infections
For neurosyphilis, due to the poor penetration of benzathine penicillin into the central nervous system, those affected are given large doses of intravenous penicillin G for a minimum of 10 days.[3][34] If a person is allergic to penicillin, ceftriaxone may be used or penicillin desensitization attempted.[3] Other late presentations may be treated with once-weekly intramuscular benzathine penicillin for three weeks.[3] Treatment at this stage solely limits further progression of the disease and has a limited effect on damage which has already occurred.[3] Serologic cure can be measured when the non-treponemal titers decline by a factor of 4 or more in 6–12 months in early syphilis or 12–24 months in late syphilis.[21]
Jarisch–Herxheimer reaction

One of the potential side effects of treatment is the Jarisch–Herxheimer reaction.[3] It frequently starts within one hour and lasts for 24 hours, with symptoms of fever, muscle pains, headache, and a fast heart rate.[3] It is caused by cytokines released by the immune system in response to lipoproteins released from rupturing syphilis bacteria.[72]
Pregnancy
Penicillin is an effective treatment for syphilis in pregnancy[73] but there is no agreement on which dose or route of delivery is most effective.[74]
Epidemiology


| no data <35 35–70 70–105 105–140 140–175 175–210 | 210–245 245–280 280–315 315–350 350–500 >500 |
In 2012, about 0.5% of adults were infected with syphilis, with 6 million new cases.[9] In 1999, it is believed to have infected 12 million additional people, with greater than 90% of cases in the developing world.[34] It affects between 700,000 and 1.6 million pregnancies a year, resulting in spontaneous abortions, stillbirths, and congenital syphilis.[7] During 2015, it caused about 107,000 deaths, down from 202,000 in 1990.[6][10] In sub-Saharan Africa, syphilis contributes to approximately 20% of perinatal deaths.[7] Rates are proportionally higher among intravenous drug users, those who are infected with HIV, and men who have sex with men.[12][13][14] In the United States about 55,400 people are newly infected each year as of 2014[update].[76] African Americans accounted for almost half of all cases in 2010.[77] As of 2014, syphilis infections continue to increase in the United States.[78][79] In the United States as of 2020, rates of syphilis have increased by more than threefold; in 2018 approximately 86% of all cases of syphilis in the United States were in men.[21] In 2021, preliminary CDC data illustrated that 2,677 cases of congenital syphilis were found in the population of 332 million in the United States.[80]
Syphilis was very common in Europe during the 18th and 19th centuries.[11] Flaubert found it universal among 19th-century Egyptian prostitutes.[81] In the developed world during the early 20th century, infections declined rapidly with the widespread use of antibiotics, until the 1980s and 1990s.[11] Since 2000, rates of syphilis have been increasing in the US, Canada, the UK, Australia and Europe, primarily among men who have sex with men.[34] Rates of syphilis among US women have remained stable during this time, while rates among UK women have increased, but at a rate less than that of men.[82] Increased rates among heterosexuals have occurred in China and Russia since the 1990s.[34] This has been attributed to unsafe sexual practices, such as sexual promiscuity, prostitution, and decreasing use of barrier protection.[34][82][83]
Left untreated, it has a mortality rate of 8% to 58%, with a greater death rate among males.[3] The symptoms of syphilis have become less severe over the 19th and 20th centuries, in part due to widespread availability of effective treatment, and partly due to virulence of the bacteria.[23] With early treatment, few complications result.[22] Syphilis increases the risk of HIV transmission by two to five times, and coinfection is common (30–60% in some urban centers).[3][34] In 2015, Cuba became the first country to eliminate mother-to-child transmission of syphilis.[84]
History
Origin, spread and discovery

Paleopathologists have known for decades that syphilis was present in the Americas before European contact.[86][87] The situation in Europe and Afro-Eurasia has been murkier and caused considerable debate.[88] According to the Columbian theory, syphilis was brought to Spain by the men who sailed with Christopher Columbus in 1492 and spread from there, with a serious epidemic in Naples beginning as early as 1495. Contemporaries believed the disease sprang from American roots, and in the 16th century physicians wrote extensively about the new disease inflicted on them by the returning explorers.[89]
Most evidence supports the Columbian origin hypothesis.[90] However, beginning in the 1960s, examples of probable treponematosis—the parent disease of syphilis, bejel, and yaws—in skeletal remains shifted the opinion of some towards a "pre-Columbian" origin.[91][92]
When living conditions changed with urbanization, elite social groups began to practice basic hygiene and started to separate themselves from other social tiers. Consequently, treponematosis was driven out of the age group in which it had become endemic. It then began to appear in adults as syphilis. Because they had never been exposed as children, they were not able to fend off serious illness. Spreading the disease via sexual contact also led to victims being infected with a massive bacterial load from open sores on the genitalia. Adults in higher socioeconomic groups then became very sick with painful and debilitating symptoms lasting for decades. Often, they died of the disease, as did their children who were infected with congenital syphilis. The difference between rural and urban populations was first noted by Ellis Herndon Hudson, a clinician who published extensively about the prevalence of treponematosis, including syphilis, in times past.[93] The importance of bacterial load was first noted by the physician Ernest Grin in 1952 in his study of syphilis in Bosnia.[94]
The most compelling evidence for the validity of the pre-Columbian hypothesis is the presence of syphilitic-like damage to bones and teeth in medieval skeletal remains. While the absolute number of cases is not large, new ones are continually discovered, most recently in 2015.[95] At least fifteen cases of acquired treponematosis based on evidence from bones, and six examples of congenital treponematosis based on evidence from teeth, are now widely accepted. In several of the twenty-one cases the evidence may also indicate syphilis.[96]

In 2020, a group of leading paleopathologists concluded that enough evidence had been collected to prove that treponemal disease, almost certainly including syphilis, had existed in Europe prior to the voyages of Columbus.[97] There is an outstanding issue, however. Damaged teeth and bones may seem to hold proof of pre-Columbian syphilis, but there is a possibility that they point to an endemic form of treponemal disease instead. As syphilis, bejel, and yaws vary considerably in mortality rates and the level of human disease they elicit, it is important to know which one is under discussion in any given case, but it remains difficult for paleopathologists to distinguish among them. (The fourth of the treponemal diseases is pinta, a skin disease and therefore unrecoverable through paleopathology.) Ancient DNA (aDNA) holds the answer, because just as only aDNA suffices to distinguish between syphilis and other diseases that produce similar symptoms in the body, it alone can differentiate spirochetes that are 99.8 percent identical with absolute accuracy.[98] Progress on uncovering the historical extent of syndromes through aDNA remains slow, however, because the bacterium responsible for treponematosis is rare in skeletal remains and fragile, making it notoriously difficult to recover and analyze. Precise dating to the medieval period is not yet possible but work by Kettu Majander et al. uncovering the presence of several different kinds of treponematosis at the beginning of the early modern period argues against its recent introduction from elsewhere. Therefore, they argue, treponematosis—possibly including syphilis—almost certainly existed in medieval Europe.[99]
Despite significant progress in tracing the presence of syphilis in past historic periods, definitive findings from paleopathology and aDNA studies are still lacking for the medieval period. Evidence from art is therefore helpful in settling the issue. Research by Marylynn Salmon has demonstrated that deformities in medieval subjects can be identified by comparing them to those of modern victims of syphilis in medical drawings and photographs.[100] One of the most typical deformities, for example, is a collapsed nasal bridge called saddle nose. Salmon discovered that it appeared often in medieval illuminations, especially among the men tormenting Christ in scenes of the crucifixion. The association of saddle nose with men perceived to be so evil they would kill the son of God indicates the artists were thinking of syphilis, which is typically transmitted through sexual intercourse with promiscuous partners, a mortal sin in medieval times.
It remains mysterious why the authors of medieval medical treatises so uniformly refrained from describing syphilis or commenting on its existence in the population. Many may have confused it with other diseases such as leprosy (Hansen's disease) or elephantiasis. The great variety of symptoms of treponematosis, the different ages at which the various diseases appear, and its widely divergent outcomes depending on climate and culture, would have added greatly to the confusion of medical practitioners, as indeed they did right down to the middle of the 20th century. In addition, evidence indicates that some writers on disease feared the political implications of discussing a condition more fatal to elites than to commoners. Historian Jon Arrizabalaga has investigated this question for Castile with startling results revealing an effort to hide its association with elites.[101]
The first written records of an outbreak of syphilis in Europe occurred in 1495 in Naples, Italy, during a French invasion (Italian War of 1494–98).[11][42] Since it was claimed to have been spread by French troops, it was initially called the "French disease" by the people of Naples.[102] The disease reached London in 1497 and was recorded at St Bartholomew's Hospital as infecting 10 out of the 20 patients.[103] In 1530, the pastoral name "syphilis" (the name of a character) was first used by the Italian physician and poet Girolamo Fracastoro as the title of his Latin poem in dactylic hexameter Syphilis sive morbus gallicus (Syphilis or The French Disease) describing the ravages of the disease in Italy.[104][105] In Great Britain it was also called the "Great Pox".[106][107]
In the 16th through 19th centuries, syphilis was one of the largest public health burdens in prevalence, symptoms, and disability,[108]: 208–209 [109] although records of its true prevalence were generally not kept because of the fearsome and sordid status of sexually transmitted infections in those centuries.[108]: 208–209 According to a 2020 study, more than 20% of individuals in the age range 15–34 years in late 18th-century London were treated for syphilis.[110] At the time the causative agent was unknown but it was well known that it was spread sexually and also often from mother to child. Its association with sex, especially sexual promiscuity and prostitution, made it an object of fear and revulsion and a taboo. The magnitude of its morbidity and mortality in those centuries reflected that, unlike today, there was no adequate understanding of its pathogenesis and no truly effective treatments. Its damage was caused not so much by great sickness or death early in the course of the disease but rather by its gruesome effects decades after infection as it progressed to neurosyphilis with tabes dorsalis. Mercury compounds and isolation were commonly used, with treatments often worse than the disease.[106]
The causative organism, Treponema pallidum, was first identified by Fritz Schaudinn and Erich Hoffmann, in 1905.[111] The first effective treatment for syphilis was arsphenamine, discovered by Sahachiro Hata in 1909, during a survey of hundreds of newly synthesized organic arsenical compounds led by Paul Ehrlich. It was manufactured and marketed from 1910 under the trade name Salvarsan by Hoechst AG.[112] This organoarsenic compound was the first modern chemotherapeutic agent.
During the 20th century, as both microbiology and pharmacology advanced greatly, syphilis, like many other infectious diseases, became more of a manageable burden than a scary and disfiguring mystery, at least in developed countries among those people who could afford to pay for timely diagnosis and treatment. Penicillin was discovered in 1928, and effectiveness of treatment with penicillin was confirmed in trials in 1943,[106] at which time it became the main treatment.[113]
Many famous historical figures, including Franz Schubert, Arthur Schopenhauer, Édouard Manet,[11] Charles Baudelaire,[114] and Guy de Maupassant are believed to have had the disease.[115] Friedrich Nietzsche was long believed to have gone mad as a result of tertiary syphilis, but that diagnosis has recently come into question.[116]
Arts and literature
The earliest known depiction of an individual with syphilis is Albrecht Dürer's Syphilitic Man (1496), a woodcut believed to represent a Landsknecht, a Northern European mercenary.[117] The myth of the femme fatale or "poison women" of the 19th century is believed to be partly derived from the devastation of syphilis, with classic examples in literature including John Keats' "La Belle Dame sans Merci".[118][119]
The Flemish artist Stradanus designed a print called Preparation and Use of Guayaco for Treating Syphilis, a scene of a wealthy man receiving treatment for syphilis with the tropical wood guaiacum sometime around 1590.[120]
Tuskegee and Guatemala studies

The "Tuskegee Study of Untreated Syphilis in the Negro Male" was an infamous, unethical and racist clinical study conducted between 1932 and 1972 by the U.S. Public Health Service.[121][122] Whereas the purpose of this study was to observe the natural history of untreated syphilis; the African-American men in the study were told they were receiving free treatment for "bad blood" from the United States government.[123]
The Public Health Service started working on this study in 1932 in collaboration with Tuskegee University, a historically black college in Alabama. Researchers enrolled 600 poor, African American sharecroppers from Macon County, Alabama in the study. Of these men, 399 had contracted syphilis before the study began, and 201 did not have the disease.[122] Medical care, hot meals and free burial insurance were given to those who participated. The men were told that the study would last six months, but in the end, it continued for 40 years.[122] After funding for treatment was lost, the study was continued without informing the men that they were only being studied and would not be treated. Facing insufficient participation, the Macon County Health Department nevertheless wrote to subjects to offer them a "last chance" to get a special "treatment", which was not a treatment at all, but a spinal tap administered exclusively for diagnostic purposes.[121] None of the men infected were ever told that they had the disease, and none were treated with penicillin even after the antibiotic had been proven to successfully treat syphilis. According to the Centers for Disease Control, the men were told they were being treated for "bad blood"—a colloquialism describing various conditions such as fatigue, anemia and syphilis—which was a leading cause of death among southern African American men.[122]
The 40-year study became a textbook example of poor medical ethics because researchers had knowingly withheld treatment with penicillin and because the subjects had been misled concerning the purposes of the study. The revelation in 1972 of these study failures by a whistleblower, Peter Buxtun, led to major changes in U.S. law and regulation on the protection of participants in clinical studies. Now studies require informed consent,[124] communication of diagnosis, and accurate reporting of test results.[125]

Similar experiments were carried out in Guatemala from 1946 to 1948. It was done during the administration of American President Harry S. Truman and Guatemalan President Juan José Arévalo with the cooperation of some Guatemalan health ministries and officials.[126] Doctors infected soldiers, prostitutes, prisoners and mental patients with syphilis and other sexually transmitted infections, without the informed consent of the subjects and treated most subjects with antibiotics. The experiment resulted in at least 83 deaths.[127][128] In October 2010, the U.S. formally apologized to Guatemala for the ethical violations that took place. Secretary of State Hillary Clinton and Health and Human Services Secretary Kathleen Sebelius stated "Although these events occurred more than 64 years ago, we are outraged that such reprehensible research could have occurred under the guise of public health. We deeply regret that it happened, and we apologize to all the individuals who were affected by such abhorrent research practices."[129] The experiments were led by physician John Charles Cutler who also participated in the late stages of the Tuskegee syphilis experiment.[130]
Names
Syphilis was first called grande verole or the "great pox" by the French. Other historical names have included "button scurvy", sibbens, frenga and dichuchwa, among others.[131][132] Since it was a disgraceful disease, the disease was known in several countries by the name of their neighbouring, often hostile country.[113] The English, the Germans, and the Italians called it "the French disease", while the French referred to it as the "Neapolitan disease". The Dutch called it the "Spanish/Castilian disease".[113] To the Turks it was known as the "Christian disease", whilst in India, the Hindus and Muslims named the disease after each other.[113]
References
- ^ a b c d Ghanem KG, Hook EW (2020). "303. Syphilis". In Goldman L, Schafer AI (eds.). Goldman-Cecil Medicine. Vol. 2 (26th ed.). Philadelphia: Elsevier. pp. 1983–1989. ISBN 978-0-323-55087-1.
- ^ a b c d e f g h i j k l m n "Syphilis – CDC Fact Sheet (Detailed)". CDC. 2 November 2015. Archived from the original on 6 February 2016. Retrieved 3 February 2016.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar Kent ME, Romanelli F (February 2008). "Reexamining syphilis: an update on epidemiology, clinical manifestations, and management". Annals of Pharmacotherapy. 42 (2): 226–36. doi:10.1345/aph.1K086. PMID 18212261. S2CID 23899851.
- ^ a b c d e f "Syphilis". CDC. 4 June 2015. Archived from the original on 21 February 2016. Retrieved 3 February 2016.
- ^ a b GBD 2015 Maternal Mortality Collaborators (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ^ a b c GBD 2015 Mortality and Causes of Death Collaborators (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ^ a b c d e f g h Woods, CR (June 2009). "Congenital syphilis-persisting pestilence". The Pediatric Infectious Disease Journal. 28 (6): 536–537. doi:10.1097/INF.0b013e3181ac8a69. PMID 19483520.
- ^ "Pinta". NORD. Archived from the original on 16 August 2018. Retrieved 13 April 2018.
- ^ a b Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. (2015). "Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting". PLOS ONE. 10 (12): e0143304. Bibcode:2015PLoSO..1043304N. doi:10.1371/journal.pone.0143304. PMC 4672879. PMID 26646541.
- ^ a b Lozano R (15 December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. hdl:10536/DRO/DU:30050819. PMC 10790329. PMID 23245604. S2CID 1541253. Archived from the original on 19 May 2020. Retrieved 9 April 2020.
- ^ a b c d e f Franzen C (December 2008). "Syphilis in composers and musicians – Mozart, Beethoven, Paganini, Schubert, Schumann, Smetana". European Journal of Clinical Microbiology & Infectious Diseases. 27 (12): 1151–57. doi:10.1007/s10096-008-0571-x. PMID 18592279. S2CID 947291.
- ^ a b Coffin L, Newberry A, Hagan H, Cleland C, Des Jarlais D, Perlman D (January 2010). "Syphilis in Drug Users in Low and Middle Income Countries". The International Journal on Drug Policy. 21 (1): 20–27. doi:10.1016/j.drugpo.2009.02.008. PMC 2790553. PMID 19361976.
- ^ a b Gao L, Zhang, L., Jin, Q (September 2009). "Meta-analysis: prevalence of HIV infection and syphilis among MSM in China". Sexually Transmitted Infections. 85 (5): 354–58. doi:10.1136/sti.2008.034702. PMID 19351623. S2CID 24198278.
- ^ a b Karp G, Schlaeffer, F., Jotkowitz, A., Riesenberg, K. (January 2009). "Syphilis and HIV co-infection". European Journal of Internal Medicine. 20 (1): 9–13. doi:10.1016/j.ejim.2008.04.002. PMID 19237085.
- ^ Ferri FF (2022). "Syphilis". Ferri's Clinical Advisor 2022. Philadelphia, PA: Elsevier. p. 1452. ISBN 978-0-323-75571-9.
- ^ "Syphilis". www.who.int. World Health Organization. 21 May 2024. Retrieved 16 August 2024.
- ^ White RM (13 March 2000). "Unraveling the Tuskegee Study of Untreated Syphilis". Archives of Internal Medicine. 160 (5): 585–598. doi:10.1001/archinte.160.5.585. PMID 10724044.
- ^ "Revisiting the Great Imitator, Part I: The Origin and History of Syphilis". www.asm.org. Archived from the original on 28 July 2019. Retrieved 29 July 2019.
- ^ a b c d Committee on Infectious Diseases (2006). Larry K. Pickering (ed.). Red book 2006 Report of the Committee on Infectious Diseases (27th ed.). Elk Grove Village, IL: American Academy of Pediatrics. pp. 631–44. ISBN 978-1-58110-207-9.
- ^ "STD Facts - Syphilis (Detailed)". www.cdc.gov. 23 September 2019. Archived from the original on 30 July 2018. Retrieved 15 September 2017.
- ^ a b c d Campion EW, Ghanem KG, Ram S, Rice PA (27 February 2020). "The Modern Epidemic of Syphilis". New England Journal of Medicine. 382 (9): 845–54. doi:10.1056/NEJMra1901593. PMID 32101666. S2CID 211537893.
- ^ a b c d e f g h i j k l m n o p q r s t Eccleston K, Collins, L, Higgins, SP (March 2008). "Primary syphilis". International Journal of STD & AIDS. 19 (3): 145–51. doi:10.1258/ijsa.2007.007258. PMID 18397550. S2CID 19931104.
- ^ a b c d Mullooly C, Higgins, SP (August 2010). "Secondary syphilis: the classical triad of skin rash, mucosal ulceration and lymphadenopathy". International Journal of STD & AIDS. 21 (8): 537–45. doi:10.1258/ijsa.2010.010243. PMID 20975084. S2CID 207198662.
- ^ Dylewski J, Duong M (2 January 2007). "The rash of secondary syphilis". Canadian Medical Association Journal. 176 (1): 33–35. doi:10.1503/cmaj.060665. PMC 1764588. PMID 17200385.
- ^ a b c d e f Bhatti MT (2007). "Optic neuropathy from viruses and spirochetes". Int Ophthalmol Clin. 47 (4): 37–66, ix. doi:10.1097/IIO.0b013e318157202d. PMID 18049280. S2CID 2011299.
- ^ Baughn RE, Musher DM (14 January 2005). "Secondary Syphilitic Lesions". Clinical Microbiology Reviews. 18 (1): 205–16. doi:10.1128/CMR.18.1.205-216.2005. PMC 544174. PMID 15653827.
- ^ a b c O'Byrne P, MacPherson P (28 June 2019). "Syphilis". BMJ. 365: l4159. doi:10.1136/bmj.l4159. PMC 6598465. PMID 31253629.
- ^ "Ward 86 Practice Recommendations: Syphilis". hivinsite.ucsf.edu. Archived from the original on 29 April 2019. Retrieved 29 July 2019.
- ^ Peeling RW, Mabey D, Kamb ML, Chen XS, Radolf JD, Benzaken AS (12 October 2017). "Syphilis". Nature Reviews Disease Primers. 3 (1): 17073. doi:10.1038/nrdp.2017.73. PMC 5809176. PMID 29022569.
- ^ a b c Longo DL, Ropper AH (3 October 2019). "Neurosyphilis". New England Journal of Medicine. 381 (14): 1358–63. doi:10.1056/NEJMra1906228. PMID 31577877. S2CID 242487360.
- ^ Cunningham F, Leveno KJ, Bloom SL, Spong CY, Dashe JS, Hoffman BL, Casey BM, Sheffield JS (2013). "Abortion". Williams Obstetrics. McGraw-Hill. p. 5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Nissanka-Jayasuriya EH, Odell EW, Phillips C (September 2016). "Dental Stigmata of Congenital Syphilis: A Historic Review With Present Day Relevance". Head Neck Pathol. 10 (3): 327–331. doi:10.1007/s12105-016-0703-z. PMC 4972761. PMID 26897633.
- ^ Giacani L, Lukehart SA (January 2014). "The endemic treponematoses". Clinical Microbiology Reviews. 27 (1): 89–115. doi:10.1128/CMR.00070-13. PMC 3910905. PMID 24396138.
- ^ a b c d e f g h i j Stamm LV (February 2010). "Global challenge of antibiotic-resistant Treponema pallidum". Antimicrobial Agents and Chemotherapy. 54 (2): 583–89. doi:10.1128/aac.01095-09. PMC 2812177. PMID 19805553.
- ^ Peeling RW, Mabey D, Kamb ML, Chen XS, Radolf JD, Benzaken AS (12 October 2017). "Syphilis". Nature Reviews. Disease Primers. 3: 17073. doi:10.1038/nrdp.2017.73. PMC 5809176. PMID 29022569.
- ^ a b "Transmission of Primary and Secondary Syphilis by Oral Sex --- Chicago, Illinois, 1998–2002". Morbidity and Mortality Weekly Report. CDC. 21 October 2004. Archived from the original on 5 August 2020. Retrieved 15 January 2019.
- ^ Hoyle A, McGeeney E (2019). Great Relationships and Sex Education. Taylor and Francis. ISBN 978-1-35118-825-8. Retrieved 11 July 2023.
- ^ a b "Syphilis & MSM (Men Who Have Sex With Men) - CDC Fact Sheet". Centers for Disease Control and Prevention (CDC). 16 September 2010. Archived from the original on 24 October 2014. Retrieved 18 October 2014.
- ^ G. W. Csonka (1990). Sexually transmitted diseases: a textbook of genitourinary medicine. Baillière Tindall. p. 232. ISBN 978-0-7020-1258-7. Archived from the original on 3 May 2016.
- ^ Lukehart S, Cruz AR, Ramirez LG, Zuluaga AV, Pillay A, Abreu C, et al. (2012). "Immune Evasion and Recognition of the Syphilis Spirochete in Blood and Skin of Secondary Syphilis Patients: Two Immunologically Distinct Compartments". PLOS Neglected Tropical Diseases. 6 (7): e1717. doi:10.1371/journal.pntd.0001717. ISSN 1935-2735. PMC 3398964. PMID 22816000.
- ^ Ratnam S (January 2005). "The laboratory diagnosis of syphilis". Canadian Journal of Infectious Diseases and Medical Microbiology. 16 (1): 45–51. doi:10.1155/2005/597580. PMC 2095002. PMID 18159528.
- ^ a b Farhi D, Dupin, N (September–October 2010). "Origins of syphilis and management in the immunocompetent patient: facts and controversies". Clinics in Dermatology. 28 (5): 533–8. doi:10.1016/j.clindermatol.2010.03.011. PMID 20797514.
- ^ Singh AE, Barbara Romanowski (1 April 1999). "Syphilis: Review with Emphasis on Clinical, Epidemiologic, and Some Biologic Features". Clinical Microbiology Reviews. 12 (2): 187–209. doi:10.1128/CMR.12.2.187. PMC 88914. PMID 10194456.
- ^ Cameron CE, Lukehart SA (March 2014). "Current status of syphilis vaccine development: Need, challenges, prospects". Vaccine. 32 (14): 1602–1609. doi:10.1016/j.vaccine.2013.09.053. PMC 3951677. PMID 24135571.
- ^ Cameron CE (September 2018). "Syphilis Vaccine Development". Sexually Transmitted Diseases. 45 (9S Suppl 1): S17–S19. doi:10.1097/OLQ.0000000000000831. PMC 6089657. PMID 29528992.
- ^ Koss CA, Dunne EF, Warner L (July 2009). "A systematic review of epidemiologic studies assessing condom use and risk of syphilis". Sex Transm Dis. 36 (7): 401–5. doi:10.1097/OLQ.0b013e3181a396eb. PMID 19455075. S2CID 25571961.
- ^ "Condom Fact Sheet in Brief | CDC". www.cdc.gov. 18 April 2019. Archived from the original on 26 July 2019. Retrieved 29 July 2019.
- ^ a b "Syphilis - CDC Fact Sheet". Centers for Disease Control and Prevention (CDC). 16 September 2010. Archived from the original on 16 September 2012. Retrieved 30 May 2007.
- ^ "A young man, J. Kay, afflicted with a rodent disease which has eaten away part of his face. Oil painting, ca. 1820". wellcomelibrary.org. Archived from the original on 28 July 2017. Retrieved 28 July 2017.
- ^ a b c Schmid G (June 2004). "Economic and programmatic aspects of congenital syphilis prevention". Bulletin of the World Health Organization. 82 (6): 402–9. PMC 2622861. PMID 15356931.
- ^ US Preventive Services Task F (19 May 2009). "Screening for syphilis infection in pregnancy: U.S. Preventive Services Task Force reaffirmation recommendation statement". Annals of Internal Medicine. 150 (10): 705–9. doi:10.7326/0003-4819-150-10-200905190-00008. PMID 19451577.
- ^ a b c Hawkes S, Matin, N, Broutet, N, Low, N (15 June 2011). "Effectiveness of interventions to improve screening for syphilis in pregnancy: a systematic review and meta-analysis". The Lancet Infectious Diseases. 11 (9): 684–91. doi:10.1016/S1473-3099(11)70104-9. PMID 21683653.
- ^ "Prenatal Syphilis Screening Laws". www.cdc.gov. 8 April 2019. Archived from the original on 28 July 2019. Retrieved 29 July 2019.
- ^ Phiske MM (January 2014). "Current trends in congenital syphilis". Indian Journal of Sexually Transmitted Diseases and AIDS. 35 (1): 12–20. doi:10.4103/0253-7184.132404. PMC 4066591. PMID 24958980.
- ^ Shahrook S, Mori R, Ochirbat T, Gomi H (29 October 2014). "Strategies of testing for syphilis during pregnancy". The Cochrane Database of Systematic Reviews. 2014 (10): CD010385. doi:10.1002/14651858.CD010385.pub2. PMC 11126892. PMID 25352226.
- ^ "Trends in Sexually Transmitted Diseases in the United States: 2009 National Data for Gonorrhea, Chlamydia and Syphilis". Centers for Disease Control and Prevention. 22 November 2010. Archived from the original on 4 August 2011. Retrieved 3 August 2011.
- ^ Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FA, et al. (7 June 2016). "Screening for Syphilis Infection in Nonpregnant Adults and Adolescents". JAMA. 315 (21): 2321–7. doi:10.1001/jama.2016.5824. PMID 27272583.
- ^ "National Notifiable Diseases". Public Health Agency of Canada. 5 April 2005. Archived from the original on 9 August 2011. Retrieved 2 August 2011.
- ^ Viñals-Iglesias H, Chimenos-Küstner, E (1 September 2009). "The reappearance of a forgotten disease in the oral cavity: syphilis". Medicina Oral, Patologia Oral y Cirugia Bucal. 14 (9): e416–20. PMID 19415060.
- ^ "Table 6.5. Infectious Diseases Designated as Notifiable at the National Level-United States, 2009 [a]". Red Book. Archived from the original on 13 September 2012. Retrieved 2 August 2011.
- ^ Brunner & Suddarth's textbook of medical-surgical nursing (12th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2010. p. 2144. ISBN 978-0-7817-8589-1. Archived from the original on 18 May 2016.
- ^ Hogben M (1 April 2007). "Partner notification for sexually transmitted diseases". Clinical Infectious Diseases. 44 (Suppl 3): S160–74. doi:10.1086/511429. PMID 17342669.
- ^ Desai M, Woodhall SC, Nardone A, Burns F, Mercey D, Gilson R (2015). "Active recall to increase HIV and STI testing: a systematic review" (PDF). Sexually Transmitted Infections. 91 (5): sextrans–2014–051930. doi:10.1136/sextrans-2014-051930. ISSN 1368-4973. PMID 25759476. S2CID 663971. Archived (PDF) from the original on 23 September 2020. Retrieved 18 September 2019.
- ^ John Frith. "Syphilis – Its early history and Treatment until Penicillin and the Debate on its Origins". History. 20 (4).
- ^ "Sex and syphilis". Wellcome Collection. 30 April 2019. Retrieved 22 November 2023.
- ^ G J O'Shea (1990). "Mercury as an Antisyphilitic Chemotherapeutic Agent". Journal of the Royal Society of Medicine. 83 (June 1990): 392–395. doi:10.1177/014107689008300619. PMC 1292694. PMID 2199676. S2CID 19322310.
- ^ P. I. Nixon (20 May 1916). "The Intravenous Use of Mercuric Chlorid". JAMA. LXVI (21): 1622. doi:10.1001/jama.1916.25810470004018d.
- ^ a b Center for Disease Control and Prevention (CDC). "Syphilis-CDC fact sheet". CDC. Archived from the original on 25 February 2015. Retrieved 1 March 2015.
- ^ "Syphilis guide: Treatment and follow-up". Government of Canada. 7 July 2022.
- ^ Bai ZG, Wang B, Yang K, Tian JH, Ma B, Liu Y, et al. (13 June 2012). "Azithromycin versus penicillin G benzathine for early syphilis". The Cochrane Database of Systematic Reviews (6): CD007270. doi:10.1002/14651858.CD007270.pub2. ISSN 1469-493X. PMC 11337171. PMID 22696367.
- ^ D'Eça Júnior A, Rodrigues L, Costa LC (2017). "Jarisch–Herxheimer reaction in a patient with syphilis and human immunodeficiency virus infection". Revista da Sociedade Brasileira de Medicina Tropical. 51 (6): 877–878. doi:10.1590/0037-8682-0419-2017. PMID 30517548.
- ^ Radolf, JD, Lukehart SA, eds. (2006). Pathogenic Treponema: Molecular and Cellular Biology. Caister Academic Press. ISBN 978-1-904455-10-3.
- ^ Alexander JM, Sheffield JS, Sanchez PJ, Mayfield J, Wendel GD J (January 1999). "Efficacy of treatment for syphilis in pregnancy". Obstetrics and Gynecology. 93 (1): 5–8. doi:10.1016/s0029-7844(98)00338-x. PMID 9916946.
- ^ Walker GJ (2001). "Antibiotics for syphilis diagnosed during pregnancy". The Cochrane Database of Systematic Reviews. 2010 (3): CD001143. doi:10.1002/14651858.CD001143. PMC 8407021. PMID 11686978.
- ^ "Disease and injury country estimates". World Health Organization (WHO). 2004. Archived from the original on 11 November 2009. Retrieved 11 November 2009.
- ^ "Syphilis". www.niaid.nih.gov. 27 October 2014. Archived from the original on 7 August 2019. Retrieved 7 August 2019.
- ^ "STD Trends in the United States: 2010 National Data for Gonorrhea, Chlamydia, and Syphilis". Centers for Disease Control and Prevention (CDC). 22 November 2010. Archived from the original on 24 January 2012. Retrieved 20 November 2011.
- ^ Clement ME, Okeke NL, Hicks CB (2014). "Treatment of Syphilis". JAMA. 312 (18): 1905–17. doi:10.1001/jama.2014.13259. ISSN 0098-7484. PMC 6690208. PMID 25387188.
- ^ Cantor A, Nelson HD, Daeges M, Pappas M (2016). Screening for Syphilis in Nonpregnant Adolescents and Adults: Systematic Review to Update the 2004 U.S. Preventive Services Task Force Recommendation. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Agency for Healthcare Research and Quality (US). PMID 27336106. Archived from the original on 8 March 2021. Retrieved 5 August 2019.
- ^ Paperny AM (31 March 2023). "Syphilis cases in babies skyrocket in Canada amid healthcare failures". reuters.
- ^ Francis Steegmuller (1979). Flaubert in Egypt, A Sensibility on Tour. Chicago Review Press, Incorporated. ISBN 9780897330183.
- ^ a b Kent ME, Romanelli, F (February 2008). "Reexamining syphilis: an update on epidemiology, clinical manifestations, and management". Annals of Pharmacotherapy. 42 (2): 226–36. doi:10.1345/aph.1K086. PMID 18212261. S2CID 23899851.
- ^ Ficarra G, Carlos, R (September 2009). "Syphilis: The Renaissance of an Old Disease with Oral Implications". Head and Neck Pathology. 3 (3): 195–206. doi:10.1007/s12105-009-0127-0. PMC 2811633. PMID 20596972.
- ^ "WHO validates elimination of mother-to-child transmission of HIV and syphilis in Cuba". WHO. 30 June 2015. Archived from the original on 4 September 2015. Retrieved 30 August 2015.
- ^ The Metropolitan Museum of Art Bulletin, Summer 2007, pp. 55–56.
- ^ Rothschild BM (15 May 2005). "History of Syphilis". Clinical Infectious Diseases. 40 (10): 1454–1463. doi:10.1086/429626. ISSN 1058-4838. PMID 15844068.
- ^ Baker, B. J. and Armelagos, G. J., (1988) "The origin and antiquity of syphilis: Paleopathological diagnosis and interpretation". Current Anthropology, 29, 703–738. https://doi.org/10.1086/203691. Powell, M. L. & Cook, D. C. (2005) The Myth of Syphilis: The natural history of treponematosis in North America. Gainesville, FL: University Press of Florida. Williams, H. (1932) "The origin and antiquity of syphilis: The evidence from diseased bones, a review, with some new material from America". Archives of Pathology, 13: 779–814, 931–983.1932).
- ^ Dutour, O., et al. (Eds.). (1994). L'origine de la syphilis in Europe: avant ou après 1493? Paris, France: Éditions Errance. Baker, B. J. et al. (2020) "Advancing the Understanding of Treponemal Disease in the Past and Present". Yearbook of Physical Anthropology 171: 5–41. doi: 10.1002/ajpa.23988. Harper, K. N., Zuckerman, M. K., Harper, M. L., Kingston, J. D., Armelagos, G. J. (2011) "The origin and antiquity of syphilis revisited: An appraisal of Old World Pre-Columbian evidence of treponemal infections". Yearbook of Physical Anthropology, 54: 99–133. https://doi.org/10.1002/ajpa.21613.
- ^ For an introduction to this literature see Quétel, C. (1990). History of Syphilis. Baltimore, MD: The Johns Hopkins University Press.
- ^ Hemarjata P (17 June 2019). "Revisiting the Great Imitator: The Origin and History of Syphilis". American Society for Microbiology. Retrieved 27 November 2023.
- ^ Early work includes Henneberg, M., & Henneberg, R. J. (1994), "Treponematosis in an ancient Greek colony of Metaponto, southern Italy, 580-250 BCE" and Roberts, C. A. (1994), "Treponematosis in Gloucester, England: A theoretical and practical approach to the Pre-Columbian theory". Both in O. Dutour, et al. (Eds.), L'origine de la syphilis in Europe: avant ou après 1493? (pp. 92-98; 101–108). Paris, France: Éditions Errance.
- ^ Salmon M (13 July 2022). "Manuscripts and art support archaeological evidence that syphilis was in Europe long before explorers could have brought it home from the Americas". The Conversation. Retrieved 27 November 2023.
- ^ Hudson, E. H. (1946). "A unitarian view of treponematosis". American Journal of Tropical Medicine and Hygiene, 26 (1946), 135–139. https://doi.org/10.4269/ajtmh.1946.s1-26.135; "The treponematoses—or treponematosis?" The British Journal of Venereal Diseases, 34 (1958), 22–23; "Historical approach to the terminology of syphilis". Archives of Dermatology, 84 (1961), 545–562; "Treponematosis and man's social evolution". American Anthropologist, 67(4), 885–901. doi:10.1001/archderm.1961.01580160009002. On status see also Marylynn Salmon, Medieval Syphilis and Treponemal Disease (Leeds: Arc Humanities Press), 8, 30-33.
- ^ Grin, E. I. (1952) "Endemic Treponematosis in Bosnia: Clinical and epidemiological observations on a successful mass-treatment campaign". Bulletin of the World Health Organization, 7: 11-25.
- ^ Walker, D., Powers, N., Connell, B., & Redfern, R. (2015). "Evidence of skeletal treponematosis from the Medieval burial ground of St. Mary Spital, London, and implications for the origins of the disease in Europe". American Journal of Physical Anthropology, 156, 90–101. https://doi.org/10.1002/ajpa.22630 and Gaul, J.S., Grossschmidt, K., Budenbauer, C., & Kanz, Fabian (2015). "A probable case of congenital syphilis from pre-Columbian Austria". Anthropologischer Anzeiger, 72, 451–472. DOI: 10.1127/anthranz/2015/0504.
- ^ They include Henneberg, M., & Henneberg, R. J. (1994). "Treponematosis in an ancient Greek colony of Metaponto, southern Italy, 580-250 BCE". In O. Dutour, et al. (Eds.), L'origine de la syphilis in Europe: Avant ou après 1493? (pp. 92–98). Paris, France: Éditions Errance. Stirland, Ann. "Evidence for Pre-Columbian Treponematosis in Europe". In Dutour, O., Pálfi, G., Bérato, J., & Brun, J. -P. (Eds.). (1994). L'origine de la syphilis in Europe: avant ou après 1493? Paris, France: Éditions Errance, and Criminals and Paupers: The Graveyard of St. Margaret Fyebriggate in combusto, Norwich. With Contributions from Brian Ayers and Jayne Brown. East Anglian Archaeology 129. Dereham: Historic Environment, Norfolk Museums and Archaeology Service, 2009. Erdal, Y. S. (2006). "A pre-Columbian case of congenital syphilis from Anatolia (Nicaea, 13th century AD)". International Journal of Osteoarchaeology, 16, 16–33. https://doi.org/10.1002/oa.802. Cole G. and T. Waldron, "Apple Down 152: a putative case of syphilis from sixth century AD Anglo-Saxon England". American Journal of Physical Anthropology 2011 Jan;144(1):72-9. doi: 10.1002/ajpa.21371. Epub 2010 Aug 18. PMID 20721939. Roberts, C. A. (1994). "Treponematosis in Gloucester, England: A theoretical and practical approach to the Pre-Columbian theory". In O. Dutour, et al. (Eds.), L'origine de la syphilis in Europe: avant ou après 1493? (pp. 101–108). Paris, France: Éditions Errance.
- ^ Baker, B.J. et al. (2020) "Advancing the Understanding of Treponemal Disease in the Past and Present". Yearbook of Physical Anthropology 171: 5–41. doi: 10.1002/ajpa.23988.
- ^ Fraser, C. M., Norris, S. J., Weinstock, G. M., White, O., Sutton, G. G., Dodson, R., ... Venter, J. C. (1998). "Complete genome sequence of Treponema pallidum, the syphilis spirochete". Science, 281(5375), 375–388. https://doi.org/10.1371/journal.pntd.0001832. Čejková, D., Zobaníková, M., Chen, L., Pospíšilová, P., Strouhal, M., Qin, X., ... Šmajs, D. (2012). "Whole genome sequences of three Treponema pallidum ssp. pertenue strains: yaws and syphilis treponemes differ in less than 0.2% of the genome sequence". PLoS Neglected Tropical Diseases, 6(1), e1471. doi: 10.1371/journal.pone.0015713. Mikalová, L., Strouhal, M., Čejková, D., Zobaníková, M., Pospíšilová, P., Norris, S. J., ... Šmajs, D. (2010). "Genome analysis of Treponema pallidum subsp. pallidum and subsp. pertenue strains: Most of the genetic differences are localized in six regions". PLoS ONE, 5, e15713. doi.org/10.1371/journal.pone.0015713. Štaudová, B., Strouhal, M., Zobaníková, M., Čejková, D., Fulton, L. L., Chen, L., ... Šmajs, D. (2014). "Whole genome sequence of the Treponema pallidum subsp. endemicum strain Bosnia A: The genome is related to yaws treponemes but contains few loci similar to syphilis treponemes". PLoS Neglected Tropical Diseases, 8(11), e3261. https://doi.org/10.1371/journal.pntd.0003261.
- ^ Majander, K., Pfrengle S., Kocher, A., ..., Kühnert, J. K., Schuenemann, V. J. (2020), "Ancient Bacterial Genomes Reveal a High Diversity of Treponema pallidum Strains in Early Modern Europe". Current Biology 30, 3788–3803. Elsevier Inc. doi: 10.1016/j.cub.2020.07.058.
- ^ See her Medieval Syphilis and Treponemal Disease (Leeds: Arc Humanities Press, 2022), 61-79.
- ^ Arrizabalaga, Jon. "The Changing Identity of the French Pox in Early Renaissance Castile". In Between Text and Patient: The Medical Enterprise in Medieval and Early Modern Europe, edited by Florence Eliza Glaze and Brian K. Nance, 397–417. Florence: SISMEL, 2011.
- ^ Winters A (2006). Syphilis. New York: Rosen Pub. Group. p. 17. ISBN 9781404209060. Archived from the original on 18 August 2020. Retrieved 15 September 2017.
- ^ Hidden Killers of the Tudor Home: The Horrors of Tudor Dentistry etc
- ^ Dormandy T (2006). The worst of evils: man's fight against pain: a history (Uncorrected page proof. ed.). New Haven: Yale University Press. p. 99. ISBN 978-0300113228.
- ^ Anthony Grafton (March 1995). "Drugs and Diseases: New World Biology and Old World Learning". New Worlds, Ancient Texts The Power of Tradition and the Shock of Discovery. Harvard University Press. pp. 159–194. ISBN 9780674618763.
- ^ a b c Dayan L, Ooi, C (October 2005). "Syphilis treatment: old and new". Expert Opinion on Pharmacotherapy. 6 (13): 2271–80. doi:10.1517/14656566.6.13.2271. PMID 16218887. S2CID 6868863.
- ^ Knell RJ (7 May 2004). "Syphilis in renaissance Europe: rapid evolution of an introduced sexually transmitted disease?". Proceedings: Biological Sciences. 271 (Suppl 4): S174–6. doi:10.1098/rsbl.2003.0131. PMC 1810019. PMID 15252975.
- ^ a b de Kruif, Paul (1932). "Ch. 7: Schaudinn: The Pale Horror". Men Against Death. New York: Harcourt, Brace. OCLC 11210642. Archived from the original on 28 August 2021. Retrieved 30 September 2020.
- ^ Rayment M, Sullivan AK (April 2011). ""He who knows syphilis knows medicine"—the return of an old friend". Editorials. British Journal of Cardiology. 18: 56–58. Archived from the original on 7 August 2020. Retrieved 7 April 2018.
"He who knows syphilis knows medicine" said Father of Modern Medicine, Sir William Osler, at the turn of the 20th Century. So common was syphilis in days gone by, all physicians were attuned to its myriad clinical presentations. Indeed, the 19th century saw the development of an entire medical subspecialty – syphilology – devoted to the study of the great imitator, Treponema pallidum.
- ^ Szreter S, Siena K (2020). "The pox in Boswell's London: an estimate of the extent of syphilis infection in the metropolis in the 1770s†". The Economic History Review. 74 (2): 372–399. doi:10.1111/ehr.13000. ISSN 1468-0289.
- ^ Schaudinn FR, Hoffmann E (1905). "Vorläufiger Bericht über das Vorkommen von Spirochaeten in syphilitischen Krankheitsprodukten und bei Papillomen" [Preliminary report on the occurrence of Spirochaetes in syphilitic chancres and papillomas]. Arbeiten aus dem Kaiserlichen Gesundheitsamte. 22: 527–534.
- ^ "Salvarsan". Chemical & Engineering News. Archived from the original on 10 October 2018. Retrieved 1 February 2010.
- ^ a b c d Tampa M, Sarbu I, Matei C, Benea V, Georgescu SR (15 March 2014). "Brief History of Syphilis". Journal of Medicine and Life. 7 (1): 4–10. PMC 3956094. PMID 24653750.
- ^ Hayden D (2008). Pox: Genius, Madness, and the Mysteries of Syphilis. Basic Books. p. 113. ISBN 978-0786724130. Archived from the original on 19 August 2020. Retrieved 15 September 2017.
- ^ Halioua B (30 June 2003). "Comment la syphilis emporta Maupassant | La Revue du Praticien". www.larevuedupraticien.fr. Archived from the original on 2 June 2016. Retrieved 29 November 2016.
- ^ Bernd M. "Nietzsche, Friedrich". Encyclopædia Britannica. Archived from the original on 23 July 2012. Retrieved 19 May 2012.
- ^ Eisler CT (Winter 2009). "Who is Dürer's "Syphilitic Man"?". Perspectives in Biology and Medicine. 52 (1): 48–60. doi:10.1353/pbm.0.0065. PMID 19168944. S2CID 207268142.
- ^ Hughes R (2007). Things I didn't know: a memoir (1st Vintage Book ed.). New York: Vintage. p. 346. ISBN 978-0-307-38598-7.
- ^ Wilson E (2005). Entwistle J (ed.). Body dressing ([Online-Ausg.] ed.). Oxford: Berg Publishers. p. 205. ISBN 978-1-85973-444-5.
- ^ Reid BA (2009). Myths and realities of Caribbean history ([Online-Ausg.] ed.). Tuscaloosa, AL: University of Alabama Press. p. 113. ISBN 978-0-8173-5534-0. Archived from the original on 2 February 2016.
- ^ a b Brandt AM (December 1978). "Racism and Research: The Case of the Tuskegee Syphilis Study". The Hastings Center Report. 8 (6): 21–29. doi:10.2307/3561468. JSTOR 3561468. PMID 721302. S2CID 215820823. Archived from the original on 18 January 2021. Retrieved 9 December 2020.
- ^ a b c d "Tuskegee Study – Timeline". NCHHSTP. CDC. 25 June 2008. Archived from the original on 3 September 2011. Retrieved 4 December 2008.
- ^ Reverby, Susan M. (2009). Examining Tuskegee : the infamous syphilis study and its legacy. Chapel Hill: University of North Carolina Press. ISBN 9780807833100. OCLC 496114416.
- ^ "Code of Federal Regulations Title 45 Part 46 Protections of Human Subjects 46.1.1(i)" (PDF). U.S. Department of Health and Human Services. 15 January 2009. Archived (PDF) from the original on 28 March 2016. Retrieved 22 February 2010.
- ^ "Final Report of the Tuskegee Syphilis Study Legacy Committee". University of Virginia. May 1996. Archived from the original on 5 July 2017. Retrieved 5 August 2019.
- ^ "Fact Sheet on the 1946-1948 U.S. Public Health Service Sexually Transmitted Diseases (STD) Inoculation Study". U.S. Department of Health and Human Services. n.d. Archived from the original on 25 April 2013. Retrieved 15 April 2013.
- ^ "Guatemalans "died" in 1940s US syphilis study". BBC News. 29 August 2011. Archived from the original on 1 December 2019. Retrieved 29 August 2011.
- ^ Reverby SM (3 June 2012). "Ethical Failures and History Lessons: The U.S. Public Health Service Research Studies in Tuskegee and Guatemala". Public Health Reviews. 34 (1). doi:10.1007/BF03391665.
- ^ Hensley S (1 October 2010). "U.S. Apologizes For Syphilis Experiments in Guatemala". National Public Radio. Archived from the original on 10 November 2014. Retrieved 1 October 2010.
- ^ Chris McGreal (1 October 2010). "US says sorry for "outrageous and abhorrent" Guatemalan syphilis tests". The Guardian. Archived from the original on 14 May 2019. Retrieved 2 October 2010.
Conducted between 1946 and 1948, the experiments were led by John Cutler, a US health service physician who would later be part of the notorious Tuskegee syphilis study in Alabama in the 1960s.
- ^ Grauer AL (2011). A Companion to Paleopathology. John Wiley & Sons. p. 643. ISBN 9781444345926. Archived from the original on 11 January 2022. Retrieved 23 August 2020.
- ^ Tagarelli A, Lagonia P, Tagarelli G, Quattrone A, Piro A (April 2011). "The relation between the names and designations of syphilis in the 16th century and its clinical gravity". Sexually Transmitted Infections. 87 (3): 247. doi:10.1136/sti.2010.048405. PMID 21325442. S2CID 19185641.
Further reading
- Ghanem KG, Ram S, Rice PA (February 2020). "The Modern Epidemic of Syphilis". N. Engl. J. Med. 382 (9): 845–854. doi:10.1056/NEJMra1901593. PMID 32101666. S2CID 211537893.
- Ropper AH (October 2019). "Neurosyphilis". N. Engl. J. Med. 381 (14): 1358–1363. doi:10.1056/NEJMra1906228. PMID 31577877. S2CID 242487360.
External links
- "Syphilis - CDC Fact Sheet" Centers for Disease Control and Prevention (CDC)
- UCSF HIV InSite Knowledge Base Chapter: Syphilis and HIV Archived 20 January 2013 at the Wayback Machine
- Recommendations for Public Health Surveillance of Syphilis in the United States
- Pastuszczak M, Wojas-Pelc A (2013). "Current standards for diagnosis and treatment of syphilis: Selection of some practical issues, based on the European (IUSTI) and U.S. (CDC) guidelines". Advances in Dermatology and Allergology. 30 (4): 203–210. doi:10.5114/pdia.2013.37029. PMC 3834708. PMID 24278076.
